International Niemann–Pick Disease Alliance

Update #11 on the AIDNPC Clinical Programme
(arimoclomol in treatment of Niemann-Pick disease type C)
You can download the article here
Conference call with patient organizations (27 OCTOBER ’16)
SUMMARY
- 002 Study: Recruitment into the 002 Interventional Study
- 002 Study: US sites have been announced
- 002 Study: Clinical design overview

At the latest end-of-the-month AIDNPC telephone conference hosted by the sponsor, Orphazyme ApS, the following update on the recruitment into the ‘-001’ and ‘-002’ Studies was presented. Over the last month, no more patients have enrolled in neither of the ‘-001’ and ‘-002’ Studies, leaving the total at 35 patients enrolled to date at 12 European sites. Of these, in the ‘-002’ Study, still a total of 3 patients have been enrolled to date at the following site:
Recruitment into the 002 Interventional Study
Copenhagen, Denmark
While Orphazyme reports making headway through many of the regulatory steps at each country, the main obstacles are currently getting contracts approved at individual sites and/or getting reviewed by local ethics committees. Varying calendars and deadline schedules for these also play into the timelines.
Orphazyme believes it is closest getting enrolment started at the sites in Germany, UK, and Spain.
The three patients in Denmark have recently completed their 3-month visit.
US sites have been announced
Of particular interest to the US NP-C community, Orphazyme announced that the three US sites that are being established can now be announced. The three US sites and their contact details are as follows:
- Mayo Clinic Children’s Center (Rochester, Minnesota)
- Principal investigator: Marc C. Patterson, MD
- Contact: Jaime Sorum
- +1 507 583 5418, jaime@mayo.edu
- Children’s Hospital Pittsburgh (Pittsburgh, Pennsylvania)
- Principal investigator: Maria Escolar, MD
- Contact: Michele D. Poe
- +1 412 692 9952, poe@chp.edu
- UCSF Benioff Children’s Hospital Oakland (Oakland, California)
- Principal investigator: Paul Harmatz, MD
- Contact: Jacqueline Madden
- +1 510 428 3885 ext5745, jmadden@mail.cho.org
To track enrolment status and obtain detailed contact information for individual clinical sites in the AIDNPC programme, visit www.ClinicalTrials.gov and use identifier NCT02612129.
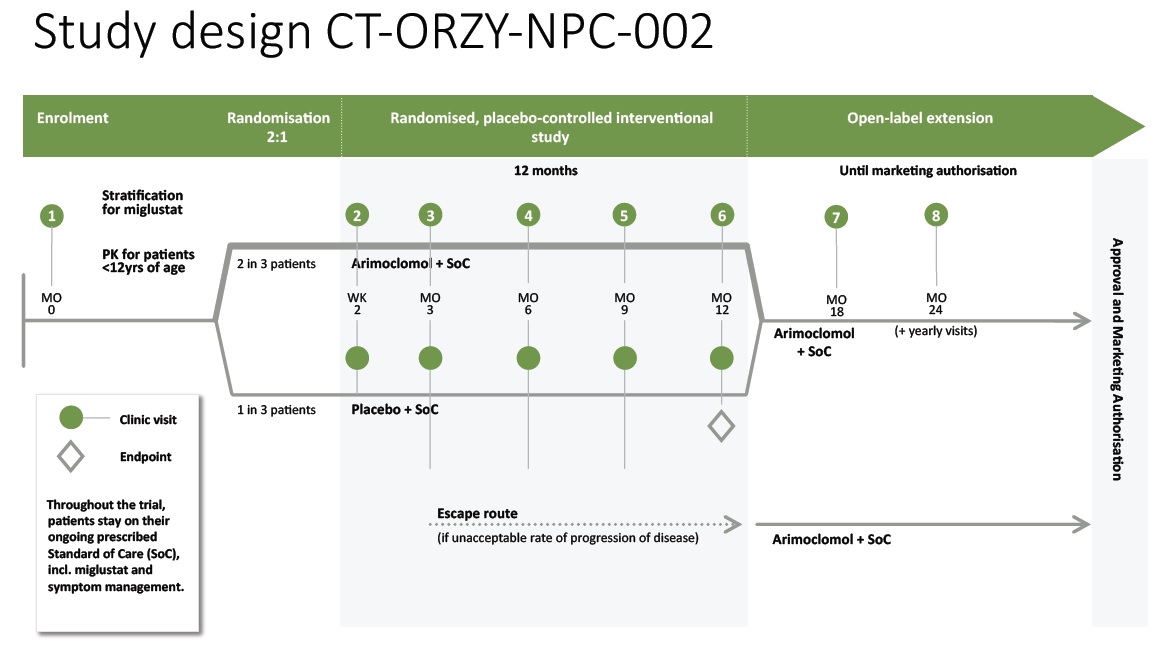
Clinical design overview
The graphic below illustrates the design of the ‘-002’ Study, including number and timing of patients visits to the sites. An escape route is provided for patients that experience an unacceptable rate of progression of the disease.
We encourage the sharing of above information with the patient community.
Next call:
The next AIDNPC call is schedule for Thursday November 24th at 15h EDT.
Visit the AIDNPC Clinical Programme website: www.AIDNPC.com