International Niemann–Pick Disease Alliance

(Available for Download in English, German, French, Spanish, Italian, and Polish)
Interim Information Bulletin
SUMMARY
- 002 Study: All patients have now started treatment with study medicine
- Orphazyme to present at Spanish NPC Association on June 24th

The AIDNPC clinical trial programme consists of two studies:
- The ‘-001’ Observational Study, which is no longer recruiting.
- The ‘-002’ Interventional Study, which is no longer recruiting.
All patients have now started treatment with study medicine. At the latest end-of-the-month AIDNPC telephone conference hosted by the sponsor, Orphazyme ApS could announce that it had met its recruitment target of 46 patients. The following sites are participating in the ‘-002’ Study:
Copenhagen, Denmark
Birmingham, UK
Great Ormond St, UK
Mainz, Germany
Münich, Germany
Paris, France
Montpellier, France
Barcelona, Spain
Warsaw, Poland
Udine, Italy
Rome, Italy
Rochester, MN-USA
Oakland, CA-USA
Today, Orphazyme is pleased to announce that all 49 patients that have entered into the ‘-002’ study have started treatment with study medication. This means that the last patient’s last visit in the blinded part of the study will take place May 30th 2018, after which database lock and data analyse can commence. As each patient has his/her last visit in the blinded part of the study, he/she will enter into an open-label extension of the study where all patients will receive only the active substance, arimoclomol, as treatment (i.e., no one will receive placebo during the open-label extension of the study).
To obtain detailed contact information for individual clinical sites in the AIDNPC programme, visit www.ClinicalTrials.gov and use identifier NCT02612129.
Orphazyme to present at Spanish NPC Association on June 24th
Orphazyme will be participating at the Scientific-Family Conference of the NPC Association of Fuenlabrada to be held on June 24th in Fuenlabrada (outside Madrid).
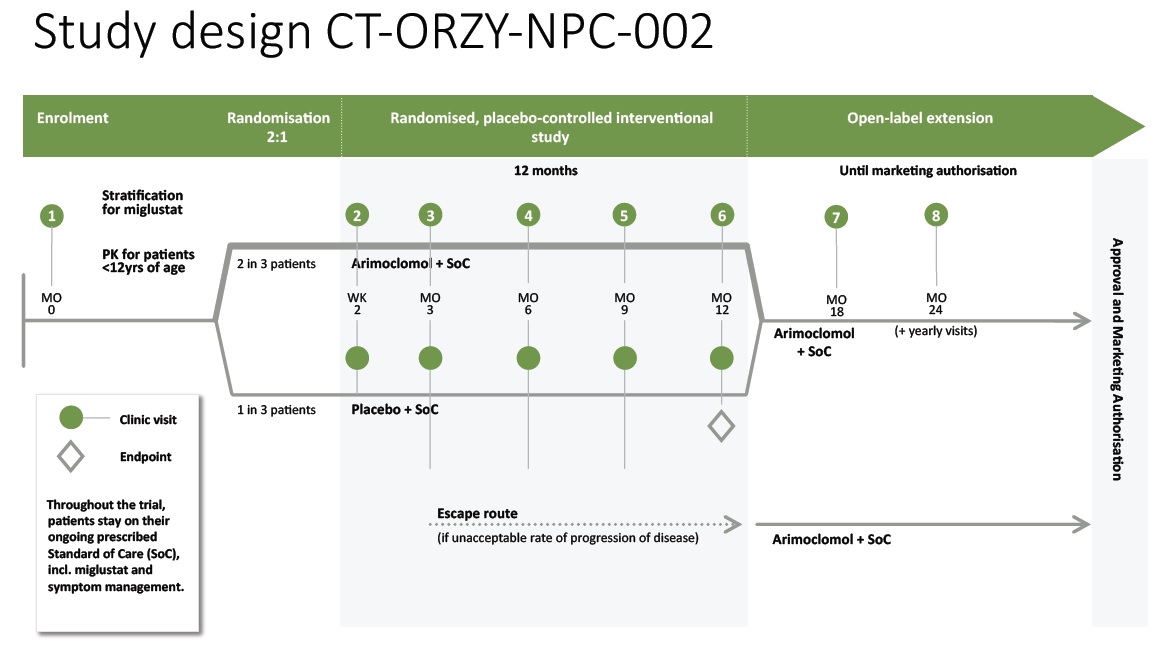
Clinical Design Overview:
The graphic below illustrates the design of the ‘-002’ Study, including number and timing of patients visits to the sites. An escape route is provided for patients that experience an unacceptable rate of progression of the disease.
We encourage the sharing of above information with the patient community.
Next call:
The next AIDNPC call is schedule for Thursday June 29th 2017 at 15h EDT.
Visit the AIDNPC Clinical Programme website: www.AIDNPC.com